EU Food Supplements Directive: Scientists Challenge Philosophy of Precaution
Two Dutch scientists, Aalt Bast of the University of Maastricht and Jaap Hanekamp of the HAN Foundation are challenging the philosophy of caution and control of the European directive that regulates food supplements, healthy products containing vitamins, minerals and other bio-ingredients useful to human health.
In their article published in Critical Reviews in Food Science and Nutrition, they charge that the real effect of the directive is not so much protection of consumer health but a kind of "secondary protection", a shield for the good repute and careers of lawmakers, administrators and scientists. What those authors of the directive have really done, according to Bast and Hanekamp, is that they covered themselves. They have 'done something', just in case anything might go wrong with supplements. Meanwhile, the good that abundant nutrient availability from vitamin and mineral supplements could be doing for public health gets all but smothered in bureaucratic red tape - threatening to achieve exactly the opposite of the publicly stated objective, which is a "a high degree of protection of consumer health".The European Food Supplements Directive has been sharply criticized by practitioners of natural medicine as well as consumers, producers and retailers of health foods. The directive has even been subject to a lawsuit challenging the validity of this legal framework for foods containing concentrated nutrients. The European Court of Justice chose to let it stand, although its own Advocate General had strongly recommended to take remedial action, calling the directive's procedures "as transparent as a black box".
Originally, the intention of this piece of legislation was to facilitate commerce of supplements in the European common market, while protecting the health of consumers. Presumably one of the objectives was to avert supposed dangers of getting "too much of a good thing". What the directive's authors have not considered is that, according to government surveys, most people don't even get the indispensable minimum of nutrients, not to mention optimal amounts for good health.
Although the directive was passed as far back as June 2002, its major issues still remain unresolved today. Limits to nutrient dosages in supplements are still under discussion by the European Commission. A decision is not expected before the end of this year. The restrictive listing of nutrient sources that may be employed as ingredients in supplements is anything but satisfying. The Alliance for Natural Health has recently filed some dossiers to test the procedure for approval of nutrients that the directive hasn't considered in its original lists.
Scientific proof of non-toxicity of ingredients that have been in use for years, some for decades, has been requested from manufacturers, but the data - clinical trials and expensive scientific assays usually made for pharmaceuticals - are hard to come by for simple nutrient substances. A final determination on the continued use of these ingredients in supplements is not expected before 2009. Temporary extensions of the status quo are slated to run out in December of that year.
Like the sword of Damocles, this directive has been threatening the freedom of choice and health of consumers, the profession of nutritional practitioners and the livelihood of health food producers and shops for close to five years now, with no resolution in sight - an unworkable political "solution" to a problem that may never have existed to begin with.
What exactly is the view of the two scientists that have now weighed in on the discussion around the restrictive philosophy of the directive an what do they propose as a way forward from this seemingly hopeless, botched situation. Here is a summary but if you are interested in the fine details, you may have to fork out some money to buy the article...
- - -
Food Supplements and European Regulation within a Precautionary Context: A Critique and Implications for Nutritional, Toxicological and Regulatory Consistency
J. C. HANEKAMP
HAN ResearchA. BAST
Mastricht University, Department of Pharmacology and ToxicologyAuthors' Abstract: In this paper, we review European legislation in the field of micronutrient food supplements and find it wanting. It is shown that the precautionary principle, embedded in European food legislation, pre-empts innovative developments in this field. In view of the scientific advances in micronutrients research, we subsequently critique the precautionary perspective and propose a novel outlook on micronutrients food supplements regulation. However, this requires a transition from the “survival” approach of the current deficiency-related RDAs to a “health-optimization” approach of a n(ew)-RDA. Genomic integrity is central in this envisioned transition.
Summary of salient points by Sepp Hasslberger:Food is essential to the maintenance, development, functioning and reproduction of human life. The major risks connected with food are microbacterial contamination (spoiled or otherwise "gone bad" foods) and an imbalance of nutrients, meaning at times we get too little or too much of some nutrient.
The food supplements directive is based on some ground-rules and ordering principles, which include a high level of consumer protection, ubiquitous availability of food; the idea that Safe Upper Limits should be established through conventional risk assessment methodology and that Maximum Permitted Levels should be developed with reference to the average dietary intake. There should be risk assessment prior to market entrance of micronutrients not yet listed on the directive's Positive Lists and rules on presenting micronutrient food supplements to the public, including directions on how to label supplements and what health claims are acceptable.
These ground-rules and principles carry distinct overtones of precaution, focussed on the risk of excess intake of micronutrients from food supplements. Even products that have been marketed for years under national laws now are subject to approval, and they may lose their status as legitimate products as part of this review process mandated by the directive.
A great problem with this approach is that it is practically impossible to demonstrate total safety. Cramer, Ford and Hall described the dilemma in 1978 in their seminal paper on the “assessment paradigm”. Their view highlights some of the values entertained by the Food Supplements Directive:
“Safety evaluation is caught in a frustrating circle. It is neither possible nor sensible to try to obtain the information needed to assess every imaginable toxic risk associated with every substance, and pursuit of greater safety therefore demands the setting of priorities as well as sensible limits for investigation. To do this with confidence requires possessing the very information that is lacking and that can be won only slowly on a few substances at a time, with significant uncertainty and at considerable cost. This requires priorities, and completes the circle of frustration.”The unremitting assessment, in other words, of increasing numbers of micronutrients or other food substances will prove prohibitive in terms of restricted research and government facilities and resources, both human and financial. More importantly, although questions of safety can be framed in scientific terms, they are not answerable by science because of these constraints.
Management options deriving from a fixation on toxicological concerns however may prove to be extremely conservative and indeed unworkable. As there is a cost to regulation, another type of cost-benefit analysis should include a look at
how much of what type of regulation generates how much health and/or prevents how much risk with the aid of how much scientific research in relation to the consumption of micronutrient food supplements.Numerous economic analyses show that beyond certain social-economic and governmental expenditures, regulation devised to enhance safety can have the opposite effect from what is intended. There are "cost-induced fatalities", which need to be included in the analysis of expected risks and benefits.
This brings up the question whether regulation and controls should be ex ante (before the fact) or ex post (after the fact). The view of the authors is that regulation that leaves a certain freedom and regulates matters ex post is definitely preferable to the directive's ex ante approach.
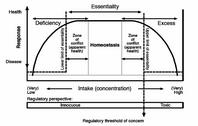
HomeostasisMicronutrients are characterized by a two-sided profile of risks and benefits. There are risks of too little and risks of too much. Generally, with micronutrients, the risks of too little outweigh those of too much. There also is a range benefit, of optimal intake - homeostasis - between the extremes of deficiency and excess.
In this graphic (click to enlarge), the authors have summarized the generalized pharmacological dose-response curve of essential micronutrients such as vitamins, minerals and other compounds. The U-shaped curve is presented in an inverted fashion. The figure does not address deficiency and excess toxicology from a regulatory or experimental point of view but centres on the organism as such, as it is exposed across a certain concentration range of micronutrients. The one-sided regulatory perspective is shown at the bottom of the image.
To further illustrate this concept of one-sided concern, the United Kingdom's "Expert Group on Vitamins and Minerals" and other bodies that have been asked to assess the risks of micronutrients, generally do not consider the benefits of micronutrients and they give at best a fleeting consideration to deficiency, while strongly concentrating on the possibilities of excess toxicology.
Genomic integrityThe integrity of the genome is a fairly new field of study. Micronutrients are a key factor in determining genomic integrity, (DNA repair, DNA synthesis and apoptosis). There is increasing evidence that higher levels of many micronutrients may be necessary for these DNA maintenance reactions and that the recommended daily amounts for some micronutrients may well be insufficient to guard against genomic instability. Selenium, polyphenols and vitamin D are concrete examples of micronutrients that are protective at levels higher than those considered "adequate".
As an overarching perspective on micronutrients, health risks are primarily related, historically, economically, and toxicologically, to deficiency. This means that there is a need to examine the precautionary principle, which is one of the pillars of the food supplements directive.
The precautionary principleThe gist of precautionary thinking is best captured in the Rio definition that is considered the most authoritative among the many formulations of the precautionary principle that can be found nowadays:
“. . . Where there are threats of serious or irreversible damage, lack of full scientific certainty shall not be used as a reason for postponing cost-effective measures to prevent environmental degradation.”However, the aspiration to prevent uncertain risks is unachievable. From a logical point of view the Rio definition, as the most authoritative of definitions, is meaningless, because the lack of scientific certainty, which is propounded to be unsolvable by the scientific method, deprives us of the possibility to calculate the costs and benefits of precautionary measures. What’s more, the problem with the precautionary principle is that it does not provide any guidance whatsoever. As Sunstein explains:
“The real problem with the Precautionary Principle . . . is that it is incoherent; it purports to give guidance, but it fails to do so, because it condemns the very steps that it requires. The regulations that the principle requires always give rise to risks of its own-and hence the principle bans what it simultaneously mandates.”Looking at the principle from this fundamental logical level, we can see its internal contradictions. In order to decide on a "safe course", new risks are evoked (from overregulation) which then evoke secondary precautionary responses, in a potentially endless chain. The only way to limit that progression of more regulation and more consequential risk, is to limit precaution. One cannot be precautious on all fronts. Total safety is unachievable, if not at the expense of total immobility, which of course defeats the purpose of precaution.
Choices need to be made. But in that case, the principle is no longer a principle but becomes a justification for choices that are made in accord with the predominant world view, resulting in policies that are blind to the negative external effects they create.
Food supplements and precautionComing back to the issue of food supplements, we see that the crucial choice (not one of safety but one of world view) has already been made. As is formulated in the Food Supplements Directive:
“An adequate and varied diet could, under normal circumstances, provide all necessary nutrients for normal development and maintenance of a healthy life in quantities which meet those established and recommended by generally acceptable scientific data. . . .”Therefore, the direction of regulation has already been "locked in" to the process.
In view of this statement in the Food Supplements Directive, food supplements are regarded as superfluous products that are, by default, only in need of excess toxicology regulation; a varied diet is more or less a guarantee for sufficient micronutrient consumption and thereby human health. The term "normal diet" begs the question of what exactly a normal diet is. The truism that we can obtain everything that we need from a balanced diet only holds if we in fact eat such a balanced diet consistently. The perspective here expounded by the EC therefore is tautological: adequate is by default adequate. How this adequacy can be achieved, and what that adequate diet would actually be like remains undiscussed. Moreover, factors impinging on the individual nutritional status are only partly related to the dietary intake on which the EC has its focus. Malabsorption (genetic or otherwise) and increased nutritional requirements (e.g. during a disease period) also greatly affect the nutritional status of individuals. However, these aspects are not considered.
Managing secondary risksWithin the precautionary context described above, the Food Supplements Directive is primarily focussed on secondary risk management. Regulators and (scientific) experts in the main are being made increasingly accountable for what they do and thereby are becoming increasingly preoccupied with managing their own risks. Particularly, secondary risks to reputation are becoming as significant as the primary risks for which policies should in fact be devised.
The increasingly dominant regulatory culture of risk-aversion therefore engenders a food supplements policy singularly focused on excess toxicity risks, while simultaneously lecturing the Europeans to “eat a normal healthy diet.” Therefore, the Directive avoids responsibility for the human health of European citizens: intoxication as a result of food supplements intake is a considerably more visible phenomenon, emphasised by the bias for negative information about possible health risks of products or activities, compared to deficiency diseases that are not (and cannot be) related to any regulatory activities (since European regulators are not responsible for the individual dietary habits of European citizens), yet have a far greater impact on public health.
It is also interesting to consider that, as the authors point out, the European market was factually deregulated in the 1980s, but that with the institutionalization of mistrust in consumer representation, the legitimacy of government market intervention was re-established and finds one of its expressions in the Food Supplement Directive.
EU Court of JusticeThe European Court of Justice, in its decision that confirmed the legitimacy of the directive, follows the same line of reasoning, however its view on micronutrients and the presumed risks involved result in an a priori selection of scientific knowledge slanted towards the precautionary principle with its institutionalized mistrust. But more importantly, it ignores one of the basic tenets of European legislation, which in the case of micronutrients is all the more ironic: "a high level of protection for human life and health".
Since it is possible to prove that a particular risk exists, but on the other hand it is quite impossible to prove the absence of any and all risk, the precautionary principle is prone to generate a probatio diabolica, a need to prove something which cannot be proved, which results in an impossibility and is therefore unlawful.
Summing up this chapter, the authors say that the current perspective of the Food Supplements Directive on micronutrients simply won’t do. The precautionary principle has no place in the debate on micronutrients because of the U-shaped curve (as presented in an inverted fashion above). Indeed, deficiency in terms of long-term health aspects (e. g. cancer and degenerative diseases) seems a much more interesting and worthwhile risk to consider when regulation is concerned. Ames is quite adamant when he states that:
“A metabolic tune-up through an improved supply of micronutrients is likely to have great health benefits, particularly for those with inadequate diets, such as many of the poor, young, obese and elderly. The issues discussed here highlight the need to educate the public about the crucial importance of nutrition and the potential health benefits of a simple and affordable daily multivitamin/mineral supplement. Tuning up metabolism to maximize human health and lifespan will require scientists, clinicians, and educators to abandon outdated models and explore more meaningful ways to prevent chronic disease and achieve optimum health. It is becoming clear that unbalanced diets will soon become the largest contributor to ill health, with smoking following close behind.”
Toward a new policy on food supplements
Since food supplements could be a relatively safe and cost-effective addition to the human diet, and considering the need to achieve a "high level of protection for human life and health", the existing body of science about nutrients and their health effects needs to be taken seriously. There should be no room for skepticism and the full weight of evidence needs to be considered, eliminating the current bias towards excess toxicity, which is contrary to the scientific method. A realistic regulatory approach needs to decide which level of public intervention is justified and necessary. This is hardly ever addressed within the "assessment paradigm". The authors quote John Stuart Mill, who said:
“Nevertheless, when there is not a certainty, but only a danger of mischief, no one but the person himself can judge of the sufficiency of the motive which may prompt him to incur the risk: in this case, therefore, (unless he is a child, or delirious, or in some state of excitement or absorption incompatible with the full use of the reflecting faculty,) he ought, I conceive, to be only warned of the danger; not forcibly prevented from exposing himself to it.”So the authors' proposal for a realistic market policy for food supplements is to put supplements into a cost-benefit context, to orient legislation in an ex-post fashion and to emphasise the possible benefits of nutrition as well as innovation in the field of nutrition. Regulation should also be market oriented, that is, create a real "level playing field" - a situation where small and medium enterprise can hold out against and compete with the big multinationals.
Intended Normal UseSupplements are consumed as a result of a conscious, individual choice to add this or that nutrient to what we eat. No one is involuntarily exposed to them, as would be the case with normal, or even added, food compounds. There is a crucial exigency, even in the current legislative context, for food business operators and indeed anyone concerned with providing or recommending supplements, to seriously consider the issues of trust, liability, product safety and consumer protection. In any case, the simple facts of household of economics will ensure that there is no involuntary excess in the consumption of micronutrients.
The pivotal concept in the proposed new orientation towards regulation of food supplements is the essential ordering principle of "intended normal use" (INU), which is the recommendation of the producer as to how much and under what circumstances to consume, as expressed on the packaging of the product. As a matter of fact, this is how things have been up until now, and the risks of this approach have proven to be extremely low, if not to say minimal. It is therefore proposed that food supplements should be allowed on the market without setting maximum or minimum levels of content. RDAs should play a primary role in the presentation of the product, together with an SUL (safe upper level), where there are specific and serious safety concerns.
An ex post approach to regulation, where safety is tackled on the basis of prevention on the basis of verifiable scientific data, would stimulate both innovation and a level playing field for companies. Restrictive listing of allowable ingredients per the current "no-data-no-market" strategy is contrary to innovation and by implication contrary to goals of better public health.
Since nutrients are an important factor in the reduction of disease risk, it is likely that, in the future, different nutrient requirements from today's RDAs may be established that take into account long term benefits of optimal nutrient intake such as cancer prevention and a reduction of the effects of aging. The authors call this an n-RDA (new RDA) concept and recommend that public health be monitored in relation to the intake of micronutrient food supplements to reveal patterns of intake, associated risks and potential benefits.
The CONCLUSIONS summarize the paper as follows:As an opening remark we surmise that in relation to the benefits and risks of micronutrients, it seems clear that concerning the “assessment paradigm” implicitly expounded by the Food Supplements Directive, the significance of science, as a means to address issues of health and safety, has been inflated out of proportion. It wants to, among other things, address trans-scientific issues (value-judgements) through the scientific method, which is unachievable.
Policies directed at human health, should by definition be wary of the set goals, and the possibilities science and regulation have to offer. Usefulness of regulation is central here. The European Food Supplements Directive has at its fundamental goal the “high level of protection for human life and health”, which, however, is specifically translated in an asymmetric precautionary fashion; only excess toxicity is addressed. This then
immediately shows the critical flaw, as risks are on all sides of the regulatory equation. For that reason, the precautionary principle, apart from our own reservations and critiques uttered by others elsewhere, has no place in the regulatory field of micronutrient food supplements. Focus on the risks of excess toxicity with recourse to the general acceptability of precaution generates the precautionary paradox: the caution that “should” give us pause causes harm, which we should pause before permitting to occur.From a risk management perspective the Food Supplements Directive, in our view, first and foremost caters for secondary risk management inclinations (liability and reputation) by explicitly referring to the “normal diet” as a sufficient source of the required micronutrients. In so doing, micronutrient food supplementation is implicitly regarded as superfluous. Therefore, the Directive openly avoids responsibility for the human health of European citizens: intoxication as a result of food supplements intake is an infinitely more “visible” phenomenon increased by the bias for negative information about possible health risks of products or activities, compared to deficiency diseases that are not (and cannot be) related to any regulatory activities, yet have a far greater impact on public health.
The model we propose limits governmental influences on the international market. This of course carries a value-judgement, which, however, is occasioned with costs and benefits deliberations. Governments need to set out the framework of health and safety, in which intended normal use and good manufacturing practice are fundamental. Market failure, as a primary [pre]occupation of precautionary culture, is not envisioned as a major problem when considering the micronutrients risks, which lie at the deficiency mark. In fact, when merely considering the issue of household economics, people in general will not be capable or willing to personally invest in food supplements in large quantities, as the costs would be prohibitive.
Micronutrient food supplementation needs to be regulated in an ex post fashion, in which marketing objectives (supplementation of the diet) need to be clearly defined by the producer. Communication to the general public, if at all possible by an independent scientific body, could add considerably to the public's understanding of micronutrients’ health and risk issues. Finally, it is our sincere opinion that in order to genuinely serve the public through regulation, the confines of regulation and the science which it requires need to be clearly spelled out by the scientists who are responsible for the elucidation of new fields of inquiry.
Full text with references is available here:- - -
posted by Sepp Hasslberger on Wednesday April 4 2007
URL of this article:
http://www.newmediaexplorer.org/sepp/2007/04/04/eu_food_supplements_directive_scientists_challenge_philosophy_of_precaution.htm
Related ArticlesFood Supplements in Europe - What is the Problem?
The European Union has issued a Directive to regulate the commerce of food supplements, which is in the process of being implemented in the member states. If reading the referenced text does not tell you what problems this directive might bring to your ability to either buy or sell supplements in one of the European Community member states, don't feel alone. That is a problem most observers have and I... [read more]
April 21, 2004 - Sepp HasslbergerRisk Free Vitamins - How Safe is Safe Enough?
Recent legislative proposals on at least three continents have centered around the perceived need to ensure the safety of natural health products, such as supplements containing vitamins and minerals. Canada has proposed drug-style regulations for supplements. In the US, a proposal termed S 722 seeks to increase the FDA's powers to remove supplements from circulation. Australia recalled 1600 diverse health products in an unprecedented prelude to - what else -... [read more]
February 03, 2004 - Sepp HasslbergerAre RDA Nutrient Levels Safe?
RDAs or Recommended Dietary Allowances of vital nutrients such as vitamins and minerals, are being promoted by health authorities as the level of consumption at which we may feel comfortable about having "taken care of our needs" - but is that really the truth? Developed during the wartime 40s, the purpose was to identify a diet that would allow US soldiers to fight as well as those staying home to... [read more]
March 02, 2004 - Sepp HasslbergerThe Dark Side of Precaution: Preventing Prevention
The precautionary principle mandates intervention to save the environment and - most importantly - our health from degradation in the case of a pressing danger, even if all the scientific data are not yet on hand. It is invoked when we face threats from chemicals, radiation or other causes. The principle seems important, yet it is difficult to find an authoritative definition. The European Union has issued a communication in... [read more]
July 09, 2006 - Sepp HasslbergerWhere Are The Bodies? - The Exceptional Safety of Nutritional Supplements
Canadian Health Authorities are ready to regulate supplements in a similar way as pharmaceutical drugs, but resistance is rallying around a law proposal - Bill C 420 - which would clearly define and distinguish supplements from dangerous drugs, suggesting that supplements are more close to foods than medicines and should therefore be regulated in a similar way as food products. Medicines regulation could crush the supplements industry and make many... [read more]
May 17, 2005 - Sepp HasslbergerMedical system is leading cause of death and injury in US
Shocking statistical evidence is cited by Gary Null PhD, Caroly Dean MD ND, Martin Feldman MD, Debora Rasio MD and Dorothy Smith PhD in their recent paper Death by Medicine - October 2003, released by the Nutrition Institute of America. "A definitive review and close reading of medical peer-review journals, and government health statistics shows that American medicine frequently causes more harm than good. The number of people having in-hospital,... [read more]
October 29, 2003 - Sepp Hasslberger